Mpox
PAMED’s Mpox resources are available to help clinicians better identify, treat, and educate patients on Mpox.
Mpox FAQ
-
What is Mpox?
Mpox is a rare disease that is caused by infection with Mpox virus. Mpox virus belongs to the Orthopoxvirus genus in the family Poxviridae. The Orthopoxvirus genus also includes variola virus (which causes smallpox), vaccinia virus (used in the smallpox vaccine), and cowpox virus.
CDC is urging health care providers in the U.S. to be alert for patients who have rash illnesses consistent with Mpox, regardless of whether they have travel or specific risk factors for Mpox and regardless of gender or sexual orientation.
-
What are the symptoms of Mpox?
The symptoms of Mpox may change over the course of the disease. For information on signs and symptoms, refer to the CDC’s Mpox page.
-
What are the case definitions for Mpox?
CDC has established several case definitions for Mpox. For the complete list, refer to CDC’s Case Definitions for Use in the 2022 Mpox Response.
-
My patient’s Mpox test result is pending. What should I tell them to do in the interim?
Instruct patients with suspected Mpox infection to isolate themselves and avoid close contact with other people and animals, including pets. Patients who do not require hospitalization, but who are potentially infectious to others, should be isolated at home.
-
What are the key characteristics and disease stages of Mpox?
Below are visual examples of Mpox rashes.
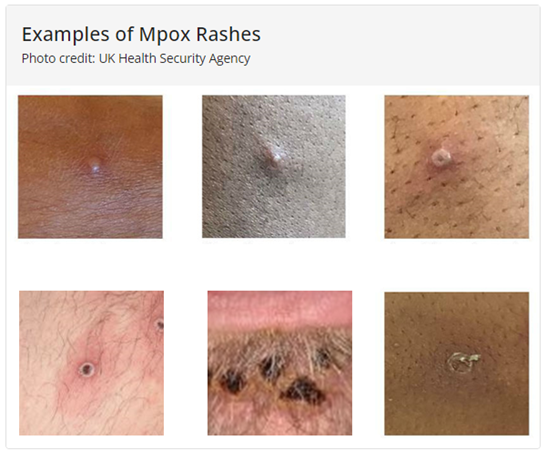
For more detailed information on identifying Mpox and the stages of the disease, refer to CDC’s Clinical Recognition.
Clinical Recognition & Diagnosis Resources
Information for Healthcare Professionals
Vaccination Basics for Healthcare Professionals
-
How should I monitor a patient who has been exposed to Mpox?
Anyone with an exposure to people or animals with mpox should monitor their health or be monitored for signs or symptoms consistent with mpox for 21 days after their last exposure. For information on monitoring, signs & symptoms, etc., refer to CDC’s Mpox Monitoring and Risk Assessment for Persons Exposed in the Community.
-
What should health care professionals do if exposed to Mpox?
The recommendations for monitoring and postexposure prophylaxis (PEP) for health care professionals are based on the assessed risk. CDC has created a table of risk level categories and associated levels of monitoring and PEP.
Risk Assessment & Exposure Resources
Mpox Monitoring and Risk Assessment for Persons Exposed in the Community
Infection Prevention and Control of Mpox in Healthcare Settings
-
How is Mpox transmitted?
Mpox can spread in different ways. It can spread through close or intimate contact with an infected person, touching objects that have been used by an infected person, from an infected mother to a fetus during pregnancy or newborn during and after birth, and from animals infected with Mpox.
For more information on modes of transmission, when an infected person can spread Mpox, and other related information, refer to CDC’s How It Spreads.
-
How can Mpox be prevented?
The following steps can be taken to protect yourself from getting Mpox:
- Get vaccinated.
- Learn steps to take to lower the risk of Mpox during sex or at a social gathering.
- Avoid close, skin-to-skin contact with people who have a rash that looks like Mpox.
- Avoid contact with objects and materials that a person with Mpox has used.
- Wash your hands often.
For more detailed information, refer to CDC’s How to Protect Yourself.
-
How is testing for Mpox conducted?
Specimen collection, storage, and shipping of human specimens are subject to Clinical Laboratory Improvement Amendments (CLIA) restrictions. Therefore, it is recommended to contact the laboratory testing facility to determine their specific requirements.
For additional information, refer to CDC’s Guidelines for Collecting and Handling Specimens for Mpox Testing.
Several commercial laboratories (i.e., Aegis Science, Labcorp, Mayo Clinic Laboratories, Quest Diagnostics and Sonic Healthcare) and the PA Department of Health (DOH) Bureau of Laboratories are offering testing for Mpox. Testing through the DOH lab requires approval from the DOH Bureau of Epidemiology (717-787-3350) or your local health department prior to submitting specimens.
DO NOT SUBMIT SPECIMENS UNTIL APPROVAL FOR TESTING IS GRANTED.
-
Who should be tested for Mpox?
Currently, testing is only recommended if an individual has a rash consistent with Mpox.
-
Must Mpox be reported?
Consultations are available from DOH, Division of Infectious Disease Epidemiology, at 717-787-3350 or your local health department should clinicians have specific questions about the evaluation and treatment of a Mpox case. However, clinicians do not need to notify DOH of suspected or confirmed cases as results as sent directly from the labs.
-
What treatment is available for Mpox?
Patients with Mpox benefit from supportive care and pain control that is implemented early in the illness. For more information, refer to CDC’s Clinical Considerations for Pain Management of Mpox.Treatment should be considered for use in people with severe disease or those at high risk of severe disease. For information on clinical manifestations that should be considered for treatment, refer to CDC’s Interim Clinical Guidance for the Treatment of Mpox.
-
Where do I obtain vaccine?
Replace with this answer – You can call 877-PA-HEALTH for assistance or use the Mpox Vaccine Locator.
In Philadelphia, call 215-685-5488 or refer to Get Your Mpox Vaccine.
-
What vaccines are available for Mpox and who should be vaccinated?
Currently, CDC does not recommend routine immunization against Mpox for the general public. Mpox vaccine can be given as post-exposure prophylaxis (PEP) both to people with known or presumed exposure to mpox virus. Vaccine can also be given to people with certain risk factors and recent experiences that might make them more likely to have been exposed to mpox.
For more information on vaccine eligibility, the vaccines available, and vaccine administration, refer to CDC’s Vaccination Basics for Healthcare Professionals.
Vaccine/Treatment Resources
Treatment Information for Healthcare Professionals
Vaccinia Immune Globulin Intravenous Prescribing Information
Cidofovir (Vistide) Information
Brincidofovir (CMX001 or Tembexa) Prescribing Information
FDA Key Facts About Vaccines to Prevent Mpox Disease
FDA Fast Facts: JYNNEOS
JYNNEOS EUA
FDA Letter to Health Care Providers re: JYNNEOS
JYNNEOS EUA Fact Sheet for Health Care Providers
Vaccination Basics for Healthcare Professionals
Vaccination Basics
Philadelphia Get Your Mpox Vaccine
-
Should patients with Mpox be isolated?
CDC recommends that people with Mpox remain isolated at home or at another location for the duration of illness, but that might not be possible in all situations.
For more information, refer to CDC’s Isolation and Infection Control At Home.
-
Should providers use PPE when treating patients with possible Mpox?
All health care personnel who enter the exam room should use PPE to include:
Gown.
Gloves.
Eye protection.
NIOSH-approved particulate respirator equipped with N95 filters or higher.
- CDC
- PA Dept. of Health
-
Health Alerts
CDC Health Alert Update for Clinicians on Mpox in People with HIV, Children and Adolescents, and People who are Pregnant or Breastfeeding
CDC Health Alert Update for Clinicians on Testing and Treatment for Mpox
CDC Health Alert Updated Case-finding Guidance: Mpox Outbreak—United States, 2022
CDC Health Alert Mpox Virus Infection in the United States and Other Non-endemic Countries—2022
CDC Health Advisory: Severe Manifestations of Mpox among People who are Immunocompromised Due to HIV or Other Conditions
CDC Health Alert Update on Managing Mpox in Patients Receiving TherapeuticsPA Health Advisory 642 - Mpox cases diagnosed in Europe, one identified in Massachusetts
PA Health Advisory 643 – Mpox Virus Infection in the United States and Other Non-endemic Countries
PA Health Advisory 647 - Updated Recommendations for Mpox Case Identification and Testing
PA Health Advisory 649 - Addition of Commercial Labs for Mpox Virus Testing
PA Health Advisory 653 - Revised Protocols Regarding the Use of Tecovirimat (TPOXX) for the Treatment of Mpox
PA Health Advisory 657 – Mpox Testing, Vaccine, and Monitoring of Healthcare Workers After Exposure Updates
PA Health Advisory 664 - Severe Manifestations of Mpox among People who are Immunocompromised Due to HIV or Other ConditionsPhiladelphia Health Advisory – Mpox Virus Infection in the US and Other Non-Endemic Countries
Philadelphia Health Advisory – Exposure Risk Assessment and Clinical Manifestation of Mpox
Philadelphia Health Advisory – Update on Clinical Presentation and Epidemiology of Mpox in Philadelphia
Philadelphia Health Advisory – Tecovirimat Treatment for Mpox
Philadelphia Health Advisory – Pain Management Recommendations for Mpox
Philadelphia Health Advisory – Mpox Vaccination Strategy and Prioritization of First Doses
Philadelphia Health Advisory – Mpox Vaccination Update: Intradermal Injection Recommended
Philadelphia Health Advisory - Severe Manifestations of Mpox among People who are Immunocompromised Due to HIV or Other Conditions
Philadelphia Health Advisory Update – Tecovirimat Treatment for Mpox Philadelphia Health Advisory Update – Jynneos Eligibility Update
Philadelphia Health Advisory Update – Mpox Updates: Testing, Treatment, and Vaccine Updates


